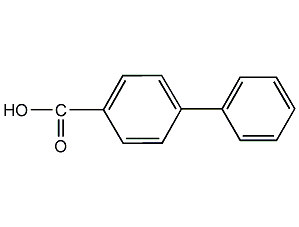
Structural formula
| Business number | 025G |
|---|---|
| Molecular formula | C13H10O2 |
| Molecular weight | 198.22 |
| label |
4-Phenylbenzoic acid, 4-biphenylcarboxylic acid, p-phenylbenzoic acid, biphenyl monocarboxylic acid, biphenyl-4-carboxylic acid, 4-Biphenylcarboxylic acid, 4-Carboxy-1,1′-biphenyl, 4-Phenylbenzic acid, 4-Carboxydiphenyl, Biphenyl-4-carboxylic acid |
Numbering system
CAS number:92-92-2
MDL number:MFCD00002553
EINECS number:202-203-1
RTECS number:DV1925100
BRN number:973519
PubChem number:24891723
Physical property data
1. Character:Colorless needle crystal-like.
2. Density (g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC): 228
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10.Autoignition point or ignition temperature (ºC): Undetermined
11.Vapor pressure (kPa,25ºC): Undetermined
12.Saturated vapor pressure (kPa,60ºC): Undetermined
13.Heat of combustion (KJ/mol): Undetermined
14.Critical temperature (ºC): Undetermined
15.Critical pressure (KPa): Undetermined
16.Oil and water (octanol/Log value of partition coefficient (water): undetermined
17.Explosion limit (%,V/V): Undetermined
18.Lower explosion limit (%,V/V): Undetermined
19.Solubility:Soluble in ethanol, ether and benzene, slightly soluble in hot water, insoluble in cold water.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 57.77
2. Molar Volume (m3/mol): 167.2
3. isotonic specific volume (90.2K):442.7
4. Surface Tension (dyne/cm):49.1
5. Polarizability(10-24cm3):22.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 211
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored in a cool place.
Synthesis method
It is obtained by reacting biphenyl with chloroacetyl and then oxidizing it with potassium permanganate. Dissolve biphenyl in benzene, slowly add anhydrous aluminum trichloride while stirring, and add chloroacetyl dropwise while slightly boiling. After adding, continue to keep warm and stir 2h. Recover the benzene until it is completely exhausted, and pour the reactant into ice water while it is hot for crystallization. Take the crystal and potassium permanganate in 90℃Following reaction, filter, neutralize with hydrochloric acid to pH is 2, and the crude product is obtained. It is then refined through ethanol recrystallization and acid-base precipitation to obtain 4-phenylbenzoic acid.
Purpose
Organic synthesis .
extended-reading:https://www.newtopchem.com/archives/1045extended-reading:https://www.newtopchem.com/archives/43920extended-reading:https://www.bdmaee.net/nt-cat-pc5-catalyst-cas3030-47-5-newtopchem/extended-reading:https://www.bdmaee.net/octyltin-oxide/extended-reading:https://www.newtopchem.com/archives/44377extended-reading:https://www.bdmaee.net/polyurethane-catalyst-pc41-catalyst-pc-41-pc41/extended-reading:https://www.bdmaee.net/nt-cat-t33-catalyst-cas11207-74-9-newtopchem/extended-reading:https://www.bdmaee.net/polyurethane-amine-catalyst-9727/extended-reading:https://www.newtopchem.com/archives/40434extended-reading:https://www.newtopchem.com/archives/40500


