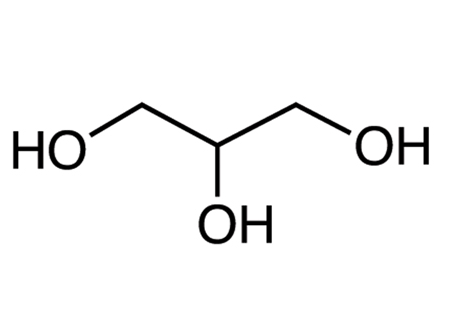
Structural formula
| Business number | 017W |
|---|---|
| Molecular formula | C3H8O3 |
| Molecular weight | 92.09 |
| label |
Glycerol, glycol, trihydroxypropane, 1,2,3-propanetriol, Glycerol, Glycol alcohol, 1,2,3-Propanetriol, Trihydroxypropane, automobile and aircraft fuel, antifreeze, hygroscopic agent, lubricants, Solvents and co-solvents, Aquasorb, carrier solvent, thickener, plasticizer, vehicle, alcohol compounds |
Numbering system
CAS number:56-81-5
MDL number:MFCD00004722
EINECS number:200-289-5
RTECS number:MA8050000
BRN number:635685
PubChem number:24895092
Physical property data
1. Properties: Colorless and odorless viscous liquid with sweet taste.
2. Boiling point (ºC, 101.3kPa): 290, 182 (2666pa)
3. Melting point (ºC, pouring point): 20
4. Relative density (g/mL, 15/15ºC): 1.26526
5. Relative density (g/mL, 20/20ºC): 1.2613
6. Relative density (g/mL, 25/25ºC): 1.26170
7. Relative vapor density (g/mL, air=1): 3.1
8. Refractive index (15ºC): 1.47547
9. Refractive index (n20ºC): 1.4746
10. Refractive index (n25ºC): 1.4730
11. Viscosity (mPa·s, 20ºC): 243
12. Viscosity (mPa·s, 25ºC): 56.0
13. Viscosity (mPa·s, 30ºC): 18
14. Viscosity (mPa·s, 50ºC): 18
15. Flash point (ºC, closed): 177
16. Flash point (ºC): 523 (on Pt); 429 (on glass)
17. Heat of evaporation (KJ/mol, 55ºC): 88.17
18. Heat of evaporation (KJ/mol, b.p.): 61.09
19.Heat of formation (KJ/mol, 15ºC, liquid): 669.05
20. Heat of combustion (KJ/mol, 25ºC, liquid): 1656.42
21. Specific heat capacity (KJ/(kg·K), 15ºC): 2.46
22. Conductivity (S/m, 20ºC): 1.0×10-8
23. Thermal conductivity (W/(m·K)): 0.29
24. Vapor pressure (kPa, 125.5ºC): 0.13
25. Body expansion coefficient (K-1): 0.000615
26. Solubility: able to absorb hydrogen sulfide, hydrocyanic acid, and sulfur dioxide. It is miscible with water and ethanol. One part of this product can be dissolved in 11 parts of ethyl acetate and about 500 parts of ether. It is insoluble in benzene, carbon disulfide, chloroform, carbon tetrachloride, petroleum ether, chloroform and oil. It is easily dehydrated and loses water to form diglycerol and polyglycerol. Oxidation produces glyceraldehyde and glyceric acid, etc. Solidifies at 0°C to form flashing rhombic crystals. Polymerization occurs at temperatures around 150°C. Incompatible with anhydrous acetic anhydride, potassium permanganate, strong acids, corrosives, fatty amines, isocyanates, and oxidants.
27. Relative density (20℃, 4℃): 1.2613
28. Relative density (25℃, 4℃): 1.255130
29. Critical temperature (ºC): 576.85
30. Critical pressure (MPa): 7.5
31. Eccentricity factor: 1.320
32. Solubility parameter (J·cm-3)0.5: 34.315
33. van der Waals area (cm2·mol-1): 7.650×1010
34. van der Waals volume (cm3·mol-1): 51.360
Toxicological data
- Toxicity Classification Poisoning
- Acute toxicity: Oral – rat LD50: 26000 mg/kg; Oral – mouse LC50: 4090 mg/kg.
- Irritation data: Skin – Rabbit 500 mg/24 hours Mild; Eyes – Rabbit 126 mg Mild.
- It is non-toxic to humans when consumed. When used as a solvent, it can be oxidized into acrolein and become irritating. The LC50 of intravenous injection in mice is 7.56g/kg, and the maximum allowable concentration in the workplace is 10mg/m3.
- Rat oral LD50: 20ml/kg; intravenous LD50: 4.4ml/kg. Store in a cool, dry place.
Ecological data
Has certain harm to water bodies. No pollution to the environment.
Molecular structure data
1. Molar refractive index: 20.51
2. Molar volume (cm3/mol): 70.9
3. Isotonic specific volume (90.2K): 199.0
4. Surface tension (dyne/cm): 61.9
5. Polarizability (10-24cm3): 8.13
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: None
6. Topological molecular polar surface area 60.7
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 25.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Colorless, transparent, odorless, viscous liquid with sweet taste and hygroscopicity. It is miscible with water, alcohols, amines and phenols in any proportion, and the aqueous solution is neutral. Soluble in 11 times of ethyl acetate and about 500 times of diethyl ether. Insoluble in benzene, chloroform, carbon tetrachloride, carbon disulfide, petroleum ether, oils, and long-chain fatty alcohols. It is flammable and can cause combustion and explosion when exposed to strong oxidants such as chromium dioxide and potassium chlorate. It is also a good solvent for many inorganic salts and gases. It is non-corrosive to metals and can be oxidized to acrolein when used as a solvent.
Chemical properties: esterification reaction with acid, such as esterification with phthalic acid to form alkyd resin. Transesterification occurs with esters. Reacts with hydrogen chloride to form chlorohydrins. There are two ways to dehydrate glycerol: intermolecular dehydration to obtain diglycerol and polyglycerol; intramolecular dehydration to obtain acrolein. Glycerol reacts with alkali to form alcoholate. Reacts with aldehydes and ketones to form acetals and ketals. Oxidation with dilute nitric acid produces glyceraldehyde and dihydroxyacetone; oxidation with periodic acid produces formic acid and formaldehyde. Contact with strong oxidants such as chromic anhydride, potassium chlorate or potassium permanganate can cause combustion or explosion. Glycerol can also play a role in nitration and acetylation.
2. Non-toxic. It is harmless even if the total amount of the dilute solution reaches 100g, and it is hydrolyzed and oxidized in the body to become a nutrient source. In animal experiments, it has the same anesthetic effect as alcohol when consumed in extremely large amounts.
3. Exists in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
4. Naturally found in tobacco, beer, wine, and cocoa.
StorageHow to save
1. Store in a clean and dry place and pay attention to sealed storage. Pay attention to moisture, water and heat protection, and it is strictly forbidden to mix with strong oxidants. Can be stored in tin-plated or stainless steel containers.
2. Packed in aluminum drums or galvanized iron drums or stored in phenolic resin-lined storage tanks. During storage and transportation, it must be protected from moisture, heat and water. It is prohibited to put glycerol together with strong oxidants (such as nitric acid, potassium permanganate, etc.). Store and transport according to general regulations on flammable chemicals.
Synthesis method
The industrial production methods of glycerol can be divided into two categories: the method using natural oils as raw materials, and the resulting glycerin is commonly known as natural glycerin; the synthesis method using propylene as raw materials, the obtained glycerin Commonly known as synthetic glycerol.
1. Production of natural glycerin. Before 1984, glycerin was all recovered from the by-products of animal and vegetable fat soap making. Until now, natural oils and fats are still the main raw materials for the production of glycerol. About 42% of the natural glycerin in the base comes from soap by-products, and 58% comes from fatty acid production. Saponification reaction of fats and oils in soap making industry. The saponification reaction product is divided into two layers: the upper layer mainly contains sodium salts of fatty acids (soap) and a small amount of glycerol, and the lower layer is waste alkali liquid, which is a dilute glycerol solution containing salts and sodium hydroxide, generally containing 9-16% of glycerol and inorganic salts. 8-20%. Grease reaction. Glycerin water (also called sweet water) obtained by hydrolysis of oil and fat has a higher glycerin content than soapmaking waste liquid, about 14-20%, and inorganic salts of 0-0.2%. In recent years, continuous high-pressure hydrolysis has been widely used. The reaction does not use a catalyst. The resulting sweet water generally does not contain inorganic acid, and the purification method is simpler than that of spent alkali. Whether it is soap-making waste liquid or glycerol water obtained by hydrolysis of oil, the amount of glycerol is not high, and they all contain various impurities. The production process of natural glycerin includes purification and concentration to obtain crude glycerol, as well as distillation, decolorization, and Deodorization refining process. This process is described in detail in some books and periodicals.

2. Production of synthetic glycerol The various pathways for synthesizing glycerin from propylene can be summarized into two major categories, namely chlorination and oxidation. Propylene chlorination method and propylene irregular acetic acid oxidation method are still used in industry.
(1) Propylene chlorination method This is the most important production method in synthetic glycerol. It includes four steps, namely high-temperature chlorination of propylene, hypochlorous acidification of chloropropene, Saponification of dichloropropanol and hydrolysis of epichlorohydrin. The hydrolysis of epichlorohydrin to glycerin is carried out at 150°C and 1.37MPa carbon dioxide pressure in an aqueous solution of 10% hydrogen oxide and 1% sodium carbonate to generate a glycerin aqueous solution containing sodium chloride with a glycerol content of 5-20%. After concentration, desalination and distillation, glycerin with a purity of more than 98% is obtained.
(2) Propylene peracetic acid oxidation method Propylene and peracetic acid react to synthesize propylene oxide, and propylene oxide isomerizes into alkene and propanol. The latter reacts with peracetic acid to generate glycidol (glycidol), which is finally hydrolyzed to glycerol. The production of peracetic acid does not require a catalyst. Acetaldehyde is oxidized with oxygen in the gas phase. Under normal pressure, 150-160°C, and a contact time of 24 seconds, the acetaldehyde conversion rate is 11% and the peracetic acid selectivity is 83%. The above-mentioned last two steps of reaction are carried out continuously in the reactive distillation tower with special structure. After the raw materials allyl alcohol and ethyl acetate solution containing peracetic acid are sent into the tower, the tower still is controlled at 60-70°C and 13-20kPa. The ethyl acetate solvent and water are evaporated from the top of the tower, and a glycerol aqueous solution is obtained from the tower still. This method has high selectivity and yield, uses peracetic acid as the oxidant, does not require a catalyst, has a fast reaction speed, and simplifies the process. The production of 1 ton of glycerin consumes 1.001t of allyl alcohol, 1.184t of peracetic acid, and 0.947t of acetic acid as a by-product. At present, the output of natural glycerin and synthetic glycerin accounts for almost 50% each, while the propylene chlorination method accounts for about 80% of Hezhi’s glycerol output. my country’s natural glycerin accounts for more than 90% of the total output.
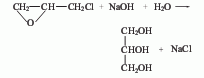
3. Dilute the industrial grade glycerin with 1/2 the amount of distilled water. After stirring thoroughly, add activated carbon and heat to 60~70℃ for decolorization, and then vacuum Filter to ensure the filtrate is clear and transparent. Control the dripping speed, and add the filtrate into the column of the pre-processed mixture of 732 strong acid cation resin and 717 strong alkali anion and cation resin to adsorb and remove electrolytes and non-electrolyte impurities such as aldehydes, pigments, and esters in glycerol.
The glycerin solution after removing impurities is distilled under reduced pressure, and the vacuum degree is controlled to be above 93326Pa. The kettle temperature is between 106 and 108°C. After steaming out most of the water, the kettle temperature is raised to 120°C for rapid dehydration. When no water comes out, the heating is stopped. The materials in the kettle are the finished products.
Purpose
1. Glycerol is an important organic chemical raw material and is widely used in many sectors of the national economy. It is an excellent hygroscopic agent, antifreeze, lubricant, solvent and co-solvent. It is an important raw material for the production of polyester, explosives, medicine, etc. In the food industry, it can be used as a water-retaining agent (for bread and cakes), a carrier solvent (for spices, pigments, and non-water-soluble preservatives), a thickener (for beverages, wine preparation, etc.), and a plasticizer. Agent(�(used in candies, desserts, meat products, etc.); can be used as a color carrier in colored foods. Glycerin is also used as a lubricant in food processing and packaging machinery. Commonly used as softeners, viscosity improvers and solvents in pharmaceutical and cosmetic manufacturing. Among polymer materials, glycerol is often used as a raw material for the production of polyurethane foam, polyether, etc. It is an important raw material for the production of alkyd resin and celluloid, and is especially used in large quantities in the manufacture of alkyd resin paint. It is also widely used in tobacco industry, ceramic industry, leather industry, wood industry and photography. and used as automobile and aircraft fuel and as antifreeze in oil fields.
2. Used as analytical reagent and gas chromatography stationary solution. Measure boron complexing agent. Used as solvents, lubricants, in the formulation of cosmetics and in the pharmaceutical industry.
3. Used as a toughening agent for polyvinyl alcohol and starch adhesives, and also used in the manufacture of unsaturated polyester resin, alkyd resin, polyester, glycerin epoxy resin, etc. As an important organic chemical raw material, it is widely used in military, food, pharmaceutical, daily chemical and other industries, with more than 1,700 uses. Defense industry: Nitroglycerin produced by the reaction of glycerin and nitric acid is an extremely sensitive explosive. Glycerin is also used as an antifreeze in aircraft fuel. Food industry: used as solvent, hygroscopic agent and color vehicle. In flavored and colored foods, glycerin helps shape the food due to its viscosity. In the rapid freezing of food, glycerin can be used as a heat transfer medium in direct contact with the food. Glycerin is also a lubricant for food processing and packaging machinery. In addition, the application of polyglycerol and polyglyceryl esters in the manufacture of crispy foods and margarine is increasing year by year. Pharmaceutical industry: used as softener, viscosity improver and solvent. Glycerol can be used as a sedative, nitroglycerin is a vasodilator in coronary spasm, etc. Daily chemical industry: additives for cosmetics, toothpaste, food flavors, anti-drying agent for tobacco. Plastic industry: used as starting agent in polyurethane foam production. Textile printing and dyeing industry: used as lubricant, moisture absorbent, anti-shrink and anti-wrinkle treatment agent, diffusing agent, penetrating agent, etc. In addition, glycerol is widely used in ceramics, photography, leather and wood industries.
4. This product is used in stainless steel polishing solution, trivalent chromium plating solution and chemical copper plating, etc.
In cyanide zinc plating, it can make the coating smooth and delicate, improve cathodic polarization, and also make the coating bright. Glycerol and triethanolamine can be used for bright nickel plating at room temperature in a certain proportion.
extended-reading:https://www.bdmaee.net/spraying-catalyst-pt1003/extended-reading:https://www.cyclohexylamine.net/tetrachloroethylene-perchloroethylene-cas127-18-4/extended-reading:https://www.bdmaee.net/wp-content/uploads/2023/02/2.jpgextended-reading:https://www.cyclohexylamine.net/ethyl-4-bromobutyrate/extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/2-6.jpgextended-reading:https://www.newtopchem.com/archives/category/products/page/152extended-reading:https://www.cyclohexylamine.net/category/product/page/13/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/33-9.jpgextended-reading:https://www.newtopchem.com/archives/674extended-reading:https://www.bdmaee.net/niax-b-18-tertiary-amine-catalyst-momentive/


